The Kitchen as Laboratory: Reflections on the Science of Food and Cooking
The Kitchen as Laboratory: Reflections on the Science of Food and Cooking
Contents
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
What Can We See? What Can We See?
-
Sugar Blends Sugar Blends
-
Blending, Tempering, Kinetics Blending, Tempering, Kinetics
-
Why Should the Glass Transition Temperature Be a Culinary Parameter? Why Should the Glass Transition Temperature Be a Culinary Parameter?
-
What is This Good For? What is This Good For?
-
Further Reading Further Reading
-
-
-
-
-
-
-
-
-
-
-
-
Twenty-four Sweet Physics: Sugar, Sugar Blends, and Sugar Glasses
-
Published:August 2013
Cite
Abstract
This chapter discusses the chemistry and physics of sugar. Sugar exhibits properties that go much beyond sweetness. For example, sugar molecules bind water molecules in a “hydrate shell”; in so doing, they increase the viscosity, especially at high concentrations, of the liquids that contain them. Sugar depresses the freezing point of water, a property that is exploited in the making of ice cream. Sugar can exist as a solid in both the crystalline and amorphous states. Granulated sugar has a crystalline structure. The sugar molecules are very much ordered with respect to one another, not unlike bricks in a wall. At the opposite end is the amorphous state, in which the molecules, randomly packed together, have no organization whatsoever. Scientists refer to solid, amorphous sugar as a glass, because its behavior is analogous to that of regular window glass—hard, brittle, and fragile. The remainder of the chapter discusses glass transition temperature and why it should be a culinary parameter.
Table sugar is ubiquitously used in desserts, baked goods, drinks, and countless other food items, mainly to make them sweet. However, sugar shows properties—not easily visible—that go much beyond sweetness. For example, sugar molecules bind water molecules in a “hydrate shell”; in so doing, they increase the viscosity, especially at high concentrations, of the liquids that contain them. Also, sugar depresses the freezing point of water, a property that is exploited in the making of ice cream (described in chapter 17). In simple terms, sugar serves many more purposes in the kitchen than the ones we know best.
One purpose is the making of sugar glass. This elaborate ornamental sugar showpiece is most commonly seen in culinary competitions and at fancy banquets. The talented pastry chefs spend hours, if not days, pulling and blowing sugar, as their complex artistic visions gradually materialize, taking the form of fragile, elaborate sculptures. This pulling and blowing process resembles the work of glassblowers; it transforms the solid sugar into what appears to be fine glass. Other sugar-based creations, which are perhaps more familiar, also play on the transformation of sugar from the granulated and solid crystalline state to the glassy state. Cotton candy—one of those treats loaded with nostalgia that takes you right back to your childhood—is the ultimate sugar glass. Cotton-candy making relies on the fact that sugar can show liquid-like behavior within a relatively wide and high temperature window. As the sugar is deposited inside the hot and fast-spinning chamber of the cotton candy machine, it melts. The liquid sugar is then spun out of the chamber in the form of a continuous, fine edible thread. Cotton candy possesses expansive surface areas that facilitate the uptake and delivery of all sorts of aromas and flavors and that cause the sudden sensation of liquefaction in the mouth. This property has made cotton candy a popular treat even in the context of high-level gastronomy.
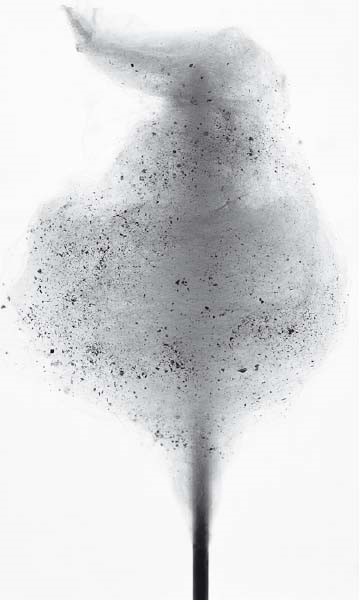
Let us take a closer look at the physical chemistry that makes sugar glass possible. Consider two basic features of sugar. First is its great deformability and its large changes in flow behavior as it melts over a wide temperature range. The second is related to the thus far unknown ability of sugar glasses to adsorb volatile aromatic compounds. To illustrate this, figure 42 shows cotton candy that has been aromatized with cubeb pepper. The culinary effect is as complex as it is sensational: sugar dissolves very quickly in the mouth, releasing the pepper’s many aromas. Almost simultaneously, the individual pepper pieces provide the sensation of heat, while emitting their complex spicy fragrance and sensations. However, something else happens: during the dissolution of the sugar filaments, the aroma compounds absorbed within seem to make their way into the retronasal region. This allows a slightly hot taste to linger in the mouth.
A fine cotton candy aromatized with cubeb pepper combines sweet and hot notes on the tongue. The sudden dissolution of the glassy sugar in the mouth and the explosive flavor release yield unusual taste sensations.
Ideally and aside from the aroma and taste-release considerations, once the cotton candy is made, it must “stay alive” for an extended period of time. This means that it should not collapse by absorbing the natural humidity of the air. The initial invention of cotton candy required some thinking about the nature of the fine sugar filaments. In particular, the sensitivity of the sugar to water had to be reduced. This leads us to two key questions: What is hidden behind these observations and developments? How can we use what is hidden to design new culinary creations?
What Can We See?
An examination into the structure of sugar before and after melting and following cooling of the melt reveals a lot about sugar’s chemistry. Granulated sugar has a crystalline structure. The sugar molecules are very much ordered with respect to one another, not unlike bricks in a wall. At the opposite end is the amorphous state, in which the molecules, randomly packed together, have no organization whatsoever. Sugar can exist as a solid in both the crystalline and amorphous states. Scientists refer to solid, amorphous sugar as a glass, because its behavior is analogous to that of regular window glass—hard, brittle, and fragile.
Sugar glasses are usually made by first melting crystalline sugar and then quickly cooling down the melt. Molten, liquid-like sugars are clear and transparent amorphous liquids that, as long as their temperatures do not exceed the caramelization point, will remain clear upon cooling. It is during cooling that a dramatic change in viscosity occurs. As the temperature decreases, the liquid becomes increasingly more viscous, until it cannot flow any more. At this point, the sugar remains very soft and flexible; with further cooling, it solidifies. It is this shift from a very soft and flexible “rubber” into a hard and brittle “glass” that polymer scientists refer to as the glass transition temperature (Tg). The temperature range in which the sugar is below the melting and above the glass transition temperature enables the creation of cotton candy and is of the utmost importance to pastry chefs—this is when they can pull and blow sugar.
Based on several experiments and tastings, we have reason to believe that the glassy state enhances the culinary sensation in a very pronounced way, far beyond sugar’s sweetness. In this context, although we have spoken only about table sugar, many other sugars show the same features; that is, they all have a glass transition. They also have small but essential differences that can make them uniquely useful for particular recipes.
Table sugar, or sucrose (saccharose), has a defined melting point, well-understood flow properties, and quickly undergoes browning or caramelization reactions upon heating. It is also, for some applications, a little too sweet. It might seem counterintuitive, however, but for many culinary applications, either in the avant-garde kitchen or in industrial food design, the aforementioned properties of sugar are actually a nuisance. Other sugars, such as isomalt and trehalose, do not show fast browning during heating, are considerably less sweet, and can better take up other tastes and aromas without overpowering them with their sweetness.
To better understand sugar glasses, we suggest the simplest of experiments: take a spoonful of table sugar, which we now know is in the crystalline state; heat it very carefully in a pan so as to avoid caramelization; and when it has completely melted, pour it on a cool plate and observe what happens. The cooled sugar will remain as transparent as a window—it has transformed itself into a sugar “glass.” Next, take this “glass” of sugar and try dissolving it in water. You will quickly realize that “glassy” sugar dissolves much faster than crystalline sugar (for pieces of similar size). For the same reason, a piece of glassy sugar will appear to deliver sweetness on the tongue more quickly than its crystalline counterpart. Seeing how we can manipulate sugar’s chemistry, it is tempting to design sugar blends with customized properties for special culinary purposes, such as a well-defined glass transition temperature, to extend the time and the temperature range in which the sugar blend can be easily pulled or blown, but also to better control its stability. The idea is then to mix different kinds of sugar with appropriate (low) sweetnesses and relatively different glass transition temperatures. Such sugar blends will show a glass transition temperature somewhere between those of the two pure components and a lower sweetness profile. This would in principle allow us to aromatize the glass without a dominating sweetness masking the flavor we intend to deliver.
What is of scientific importance when performing these experiments in the kitchen? What is important to consider when blending sugars? It should be merely a matter of mixing them at a certain ratio, melting the blend, and then cooling it. Simple? Let us look further into this process—from a physicist’s point of view.
Sugar Blends
The simplest question in this context is: If the two sugars to be blended have two different glass transition temperatures, what is the overall glass transition temperature of the mixture? This question makes sense only if we know for sure that they mix at the molecular level once we melt them together. The glass transition temperature is very much dependent on the sugar-blend composition, and the ideal blend is determined by use of the so-called Gordon-Taylor equation, which was originally proposed for polymer blends. We will not go into the mathematical details and other complicated matters; we prefer to focus on the practical aspects of sugar blending.
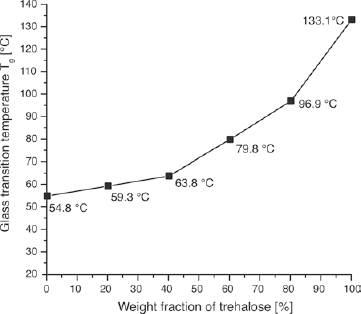
As an example, we look at the two disaccharides sucrose and trehalose, which differ in their molecular structure and, hence, show major differences in their physicochemical properties. Sucrose consists of one glucose and one fructose molecule, whereas trehalose is composed of two glucose molecules—this is why they are called disaccharides: they each possess two sugar molecules. Their glass transition temperatures and melting temperatures, measured through a technique called differential scanning calorimetry, turn out to be significantly different: the glass transition temperature is approximately 131°F (55°C) and 270°F (133°C) and the melting temperature is about 367°F (186°C) and 397°F (203°C) for sucrose and trehalose, respectively. Moreover, trehalose has the advantage over sucrose in that it is roughly half as sweet and does not undergo caramelization at the so-called soft-or hard-ball temperatures used in candy making. For reference, we present only the results for the glass transition temperature for the sucrose–trehalose blends (figure 43).
The change in the glass transition temperature of sucrose–trehalose blends as measured with the aid of a differential scanning calorimeter. The glass transition temperature changes continuously with trehalose concentration. Therefore, a precise design for gastronomic applications is possible by adjusting sweetness, flow behavior,and solidification temperature by blending.
Blending, Tempering, Kinetics
To get perfect culinary results, the mixing has to be perfect, down to the molecular level. Molecularly speaking, “perfect” mixing means that the mixture will have to show a single glass transition temperature, which reflects the true miscibility—like water and alcohol—of the two sugars. In culinary terms, perfection requires that the flow behavior of the blend be similar to that of the pure components. Since molten sugar moves slowly—think of honey, for example—it is necessary to give the blend time to mix homogeneously. A simple yet relevant experiment exquisitely illustrates this. In a coffee grinder, pulverize first the sucrose and then the trehalose. Blend them together at a 1:1 ratio in a Pyrex cup. Proceed to slowly heat up the mixture in an oil bath set to a temperature of 420°F (220°C). The blend will melt, but you will observe how sucrose melts first, followed by trehalose. A sample taken immediately after the melting of the sucrose is shown in figure 44.
Sugar filament of a 50:50 sucrose–trehalose mixture drawn immediately after melting. The filament is not homogeneous, and the lumps are showing at almost equal and characteristic distances.
We found that the flow behavior of sucrose–trehalose mixtures depends significantly on the tempering and stirring time. This manifests itself in the creation of large “pearls” during the development of the sugar filaments, as can be seen in figure 45. This indicates that on the molecular level, the sugars are not homogeneously mixed. In such a nonhomogeneous state, the blend cannot be pulled or blown in a reliable and controlled manner. Even after holding the blend for 10 minutes at a temperature in between the melting temperatures of the two sugars, the blend will not homogeneously mix. Pearls and lumps are less pronounced but still present. Only after 15 minutes can the mixture be deformed and regular fibers spun. Hence, the conclusion is very simple and general: sugars can be blended and their mechanical and flow properties designed by manipulating their concentrations and tempering times.
Sugar filaments of a 50:50 sucrose–trehalose blend after different tempering times. The two pure-component filaments are shown: (farthest left) sucrose and (farthest right) trehalose. The three other filaments represent the blend right after melting: 10 minutes and 15 minutes tempering time (left to right). The sucrose content is responsible for the light caramelization.
Now let us explore how the nature of the solid state (glassy or crystalline) influences the aromatization of sugars. This is a widely open and unexplored field and very relevant for many culinary applications. Again, this is another situation in which the fundamental questions in physics and chemistry meet at the point of flavor, in which visible length scales are addressed down to the size of molecules. A prominent example is found in chocolate. The glassy sugars used in chocolate may allow for the uptake of more aroma compounds than do crystalline sugars. Because of the disordered nature of the amorphous state, the adsorption and diffusion of aroma compounds on amorphous sugar surfaces is hypothesized to be much better than that of crystalline sugars, in which a highly ordered structure hinders the ability of the sugar to interact with its environment.
How can this be visualized? Roughly speaking, a sugar crystal has a highly regular and ordered surface. This gives it a very well-defined adsorption and desorption energy in relation to aroma compounds, which serves to restrict their interaction. Whereas the amorphous structure of sugar in a glassy state allows the aroma compounds to latch on to edges, wedges, and other irregular topographical features. Such irregularities create more opportunities for the aroma compounds to make contact with the surface. This multifaceted interaction between aroma compounds and sugar surfaces is essential to achieving a higher concentration of flavors in any finished dish.
Why Should the Glass Transition Temperature Be a Culinary Parameter?
In the presence of molten liquid sugar, small droplets of aroma compounds (which are mostly hydrophobic) reside at the surface. As we stir the sugar blend, we help emulsify, or disperse, these droplets within the molten sugar. Physics tells us that these emulsified droplets generally will want to associate with one another to form larger droplets and minimize their surface energy. However, as the blend cools, its viscosity increases and, eventually, it solidifies into a glass that contains droplets trapped in a random environment, and these droplets cannot escape from within the glassy sugar matrix. The glassy sugar thus ends up being highly aromatized with hydrophobic aroma compounds. Knowledge of the sugar’s transition temperature plays an essential role in the design of these systems: the lower the sugar’s transition temperature, the better the aromatization obtained because more time is allowed for mixing. Cooler temperatures also mean a smaller loss of volatiles.
So, what is the best sugar for making glasses, keeping in mind that they have to keep their shape and properties under humid and hot conditions, such as those in a restaurant? We know that the transition temperature of the blend should be as high as possible. A sufficiently high transition temperature ensures that there will be low-motion sugar molecules in the amorphous matrix at room temperature and thus a lower water solubility. This means that the water molecules dissolved in the air cannot easily penetrate the glass and soften it. Adding increasing amounts of trehalose to sucrose thus helps stabilize the candy (see figure 43). The sugar must have a balanced transition temperature to enable enough aromatization with the maximum stability.
What is This Good For?
It is good for gastronomy, of course. Take, for example, a blend of not very sweet sugars, such as isomalt, lactose, and trehalose. Mix in finely ground salt, aromatize with very dry lemon powder, and melt the blend as described earlier. Once melted, dip the tip of a spoon into the molten blend and pull it slowly upward to form a thread or filament. The thread will soon solidify (sooner with increasing trehalose content). You can then add this filament to steamed sea fish as a crispy component. This, of course, is also possible with common table sugar, but table sugar will more often than not caramelize, which will impart a flavor you might not want to pair with fish.
Another example is the now-famous olive oil bonbon served at José Andrés’s Minibar in Washington, D.C. Approximately 3 ounces (100 g) of crystalline isomalt, a disaccharide composed of one glucose molecule and one mannitol molecule, is melted so as to render it a viscous liquid. Dipped into the melted isomalt is a ½-inch (1.25 cm) plastic ring (wide enough to avoid burned fingers). It is quickly pulled out from the pot, causing a film of isomalt to form at the bottom of the ring. It is cooled for about 10 seconds to allow the isomalt to thicken and, immediately, with the help of a spoon, 3 milliliters or so of olive oil are poured from the opposite end of the ring. The oil pulls down the film of isomalt, which, as it cools, will form a solid, amorphous, drop-shaped casing with a long tail that contains the liquid olive oil. The bottom of the drop is lightly moistened and dipped into a mix of Maldon salt and powdered vinegar.1
We never know who the sous-chef really is then: physics or chemistry. It does not matter—what matters is the chef’s taste and the incorporation of science as a deliciously smart spice.
Further Reading
Cammenga, H. K., K. Hopp, K. Gehrich, and G. Ziegleder.
Seo, J.-A., J. Oh, H. K. Kim, Y. H. Hwang, Y. S. Yang, and S. J. Kim.
Vilgis, Thomas.
| Month: | Total Views: |
|---|---|
| October 2022 | 1 |
| December 2022 | 2 |
| January 2023 | 2 |
| February 2023 | 3 |
| March 2023 | 4 |
| April 2023 | 1 |
| May 2023 | 1 |
| June 2023 | 1 |
| July 2023 | 2 |
| August 2023 | 2 |
| September 2023 | 2 |
| October 2023 | 3 |
| November 2023 | 2 |
| December 2023 | 1 |
| February 2024 | 3 |
| April 2024 | 1 |